Did you know the percentage of oxygen in the atmosphere is a constant 21% regardless of altitude? Well it’s true! So you may be wondering why then that it gets harder to breath as you increase in altitude. The simple answer is quantity and pressure. As you increase in altitude, the quantity and pressure of atmosphere decreases.
The atmosphere around earth is comprised of gases: 78% nitrogen, 21% oxygen, and 1% various other gases like argon and carbon dioxide. We commonly refer to all these gases that make up the atmosphere as air, fresh crisp air! Out of all these gases, oxygen is the most important to the human body and is vital to all living things. Without the correct amount of oxygen in the human body a person will become sluggish, both physically and mentally, and eventually lose consciousness. We refer to this as hypoxia.
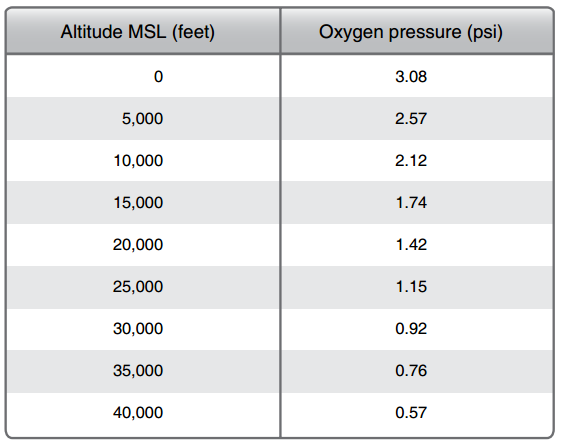
So why is it hard to breathe the higher we go? As altitude increases, the total quantity of each gas reduces; however, the proportions remain the same. This is true up to about 50 miles above the surface. You may have learned in you flight training that as altitude increases, pressure decreases. You can see this by doing a simple experiment with a bag of chips. If you were to bring a bag of chips with you over a mountain pass or on an airplane you will notice that as you go up the bag begins to expand. The bag is not expanding because it is filling up with more air but rather because there is less pressure allowing the air already inside the sealed bag to expand, and yes it will eventually pop if you go high enough. The table below depicts the pressure per square inch at particular altitudes.
The human body takes in oxygen through the lungs, which in turn saturates the blood. From the table above you can see that at sea level (0 feet) the amount of pressure is 3.08 pounds per square inch (psi). This corresponds directly to the pressure within our lungs and is sufficient enough to saturate the blood allowing us to function normally. As we climb, the pressure within the lungs will eventually reach a point that no longer allows for proper saturation of oxygen into the blood stream leading to hypoxia.
For most people, altitudes below 7,000 feet MSL will provide sufficient oxygen quantities and pressure for sufficient saturation. Once we get above 7,000 feet MSL, quantities and pressure become increasingly insufficient and at 10,000 feet MSL oxygen saturation of the blood is at 90% normal. The Aeronautical Information Manual (AIM) recommends using supplemental oxygen above 5,000 feet when flying at night. This recommendation is because decreased oxygen levels will tend to affect your night vision at a greater capacity then vision during daylight hours.
The FAA has established strict regulations for the requirement of supplemental oxygen. As long as you remain healthy and use supplemental oxygen as mandated in 14 CFR Part 91 you should have nothing to worry about.
If you have some free time, you can Google or YouTube oxygen deprivation chambers and get an idea of what happens to the body when deprived of oxygen. You may find some comical videos but in reality it is extremely serious and sometimes a fatal situation for pilots.
Quick recap on why it becomes harder to breath as altitude increases:
- Quantity of oxygen in the atmosphere decreases with altitude.
- Pressure decreases as altitude increases, making it harder for oxygen to be delivered into the bloodstream.